Investigating phase separation properties of chromatin-associated proteins using gradient elution of 1,6-hexanediol
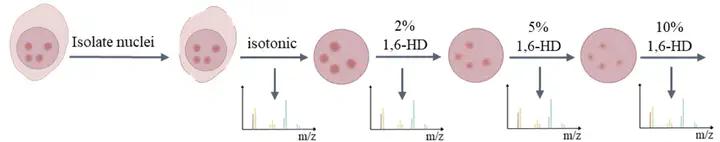 Image credit: Chao Hou
Image credit: Chao HouAbstract
Chromatin-associated phase separation proteins establish various biomolecular condensates via liquid-liquid phase separation (LLPS), which regulates vital biological processes spatially and temporally. However, the widely used methods to characterize phase separation proteins are still based on low-throughput experiments, which consume time and could not be used to explore protein LLPS properties in bulk. By combining gradient 1,6-hexanediol (1,6-HD) elution and quantitative proteomics, we developed chromatin enriching hexanediol separation coupled with liquid chromatography-mass spectrometry (CHS-MS) to explore the LLPS properties of different chromatin-associated proteins (CAPs). First, we found that CAPs were enriched more effectively in the 1,6-HD treatment group than in the isotonic solution treatment group. Further analysis showed that the 1,6-HD treatment group could effectively enrich CAPs prone to LLPS. Finally, we compared the representative proteins eluted by different gradients of 1,6-HD and found that the representative proteins of the 2% 1,6-HD treatment group had the highest percentage of IDRs and LCDs, whereas the 10% 1,6-HD treatment group had the opposite trend. This study provides a convenient high-throughput experimental method called CHS-MS. This method can efficiently enrich proteins prone to LLPS and can be extended to explore LLPS properties of CAPs in different biological systems.
Supplementary notes can be added here, including code, math, and images.